AT A GLANCE
EPRS | European Parliamentary Research Service
Author: Jana Titievskaia; Graphic: Samy Chahri, Members' Research Service
PE 690.649 – September 2021
EN
World Trade Organization TRIPS waiver
to tackle coronavirus
The coronavirus pandemic has rekindled the global debate on whether the multilateral trade regime for
intellectual property rights (IPR) protection limits access to essential medical products. Despite embedded
flexibilities in the World Trade Organization (WTO) Agreement on Trade-related Intellectual Property Rights
(TRIPS), India and South Africa, co-sponsored by a large number of developing countries, submitted an initial
proposal for a temporary waiver in response to Covid-19 in October 2020, followed by a revised proposal in May
2021, which continues to divide opinion. The US administration voiced its support for a vaccines waiver. EU
leaders indicated an openness to discussion, while putting forward an alternative plan with a focus on limiting
export restrictions, compulsory licensing and using the existing TRIPS flexibilities.
Background
The WTO TRIPS Agreement has, since 1995, set the minimum standard of protection of, inter alia, co py right,
trademarks, geographical indications, industrial designs, and trade secrets. TRIPS also includes flexibilities
to depart from the IPR protection requirements, including in the case of a health emergency. Fo r example,
members can do
compulsory licensing, which authorises an entity to produce a patented product or
process without the consent of the patent owner. In this way, members can produce generic copies for the
domestic market (but not for export), and implement fast-track procedures in health emergencies. In
addition, the
Implementation Decision of the Doha Declaration on TRIPS and Public Health (2001) allows
producing members to adopt a compulsory licence for the production of pharmaceuticals for export. This
provision enabled Canada to make and export a generic version of a patented AIDS drug to
Rwanda, which
lacked the manufacturing capacity, in 2007-2009. However, TRIPS flexibilities have been criticised as
burdensome and legally challenging.
The WTO Agreement enables members to waive an obligation in exceptional circumstances by consensus-
based decision-making; in the past, members have successfully agreed to waive specific articles o f t he TRIPS
Agreement with respect to pharmaceutical products and least-developed countries (LDCs). In
October 2020, India and South Africa made a landmark
proposal to waive several sections of the TRIPS
Agreement, to address the prevention, containment and treatment of Covid-19. The waiver would enable
members to not grant or enforce patents (section 5) or other IP obligations on copyright (section 1),
industrial designs (section 4), and the protection of undisclosed information (section 7) related to Covid-19
products and technologies. The revised
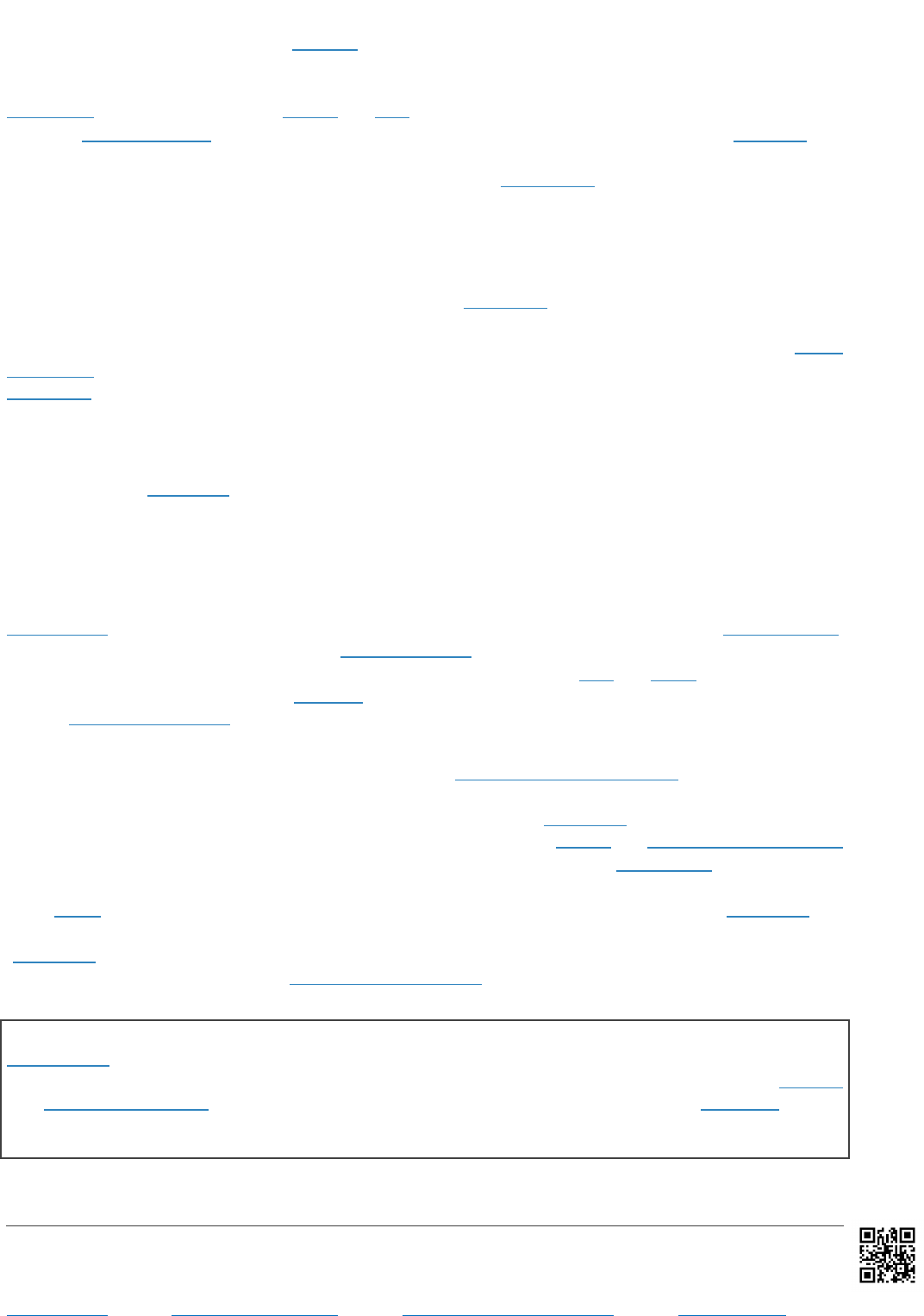
proposal of 25 May 2021 was supported
by a number of members in Africa, Asia
and Latin America (see Figure 1), and
specified the scope would apply to
health products and technologies. These
include diagnostics, therapeutics,
vaccines, medical devices and personal
protective equipment to tackle Covid-
19. The waiver would be in force for a
minimum of three years, and be
reviewed annually. LDC members are
exempt from applying most of the TRIPS
Agreement until July 2034 or until their
graduation from the LDC category.
This is an updated edition of an 'At a glance' note published in June 2021.
Figure 1 – World map of WTO members in favour of waiver
Source: Revised proposal for TRIPS waiver (May 2021).
EPRS World Trade Organization TRIPS waiver to tackle coronavirus
This document is prepared for, and addressed to, the Members and staff of the European Parliament as background material to assist them in their
parliamentary work. The content of the document is the sole responsibility of its author(s) and any opinions expressed herein should not be taken
to represent an official position of the Parliament. Reproduction and translation for non-commercial purposes are authorised, provided the source
is acknowledged and the European Parliament is given prior notice and sent a copy. © European Union, 2021.
eprs@ep.europa.eu (contact) http://www.eprs.ep.parl.union.eu (intranet) http://www.europarl.europa.eu/thinktank ( internet) http://epthinktank.eu ( blo g)
EU position
In May 2021, the Council of the EU decided to support the extension of LDCs' transitional period for the
implementation of the TRIPS Agreement by another ten years, or until the member graduates from LDC
status. With regard to the temporary TRIPS waiver, notably Germany, Portugal, Estonia and Belgium are
reportedly reserved, while Greece, France and Italy are somewhat supportive. On 4 June, the Commission
issued a communication to the WTO on TRIPS and Covid-19, reiterating the alternative proposal that
focuses on compulsory licensing, limiting export restrictions and expanding production, rather than on
waiving patent rights. On 18 June 2021, the Council adopted
conclusions on the role of IP in tackling the
Covid-19 pandemic. The Council highlighted the EU’s engagement in the WTO and its readiness to find
pragmatic approaches, such as patent pooling, licensing, and knowledge sharing platforms, and that it
stands ready to discuss other flexibilities in the TRIPS agreement.
European Parliament position
During its June I plenary session, Parliament adopted a resolution on meeting the Covid-19 challenge,
calling for support for text-based negotiations of a temporary TRIPS waiver in order to enhance global
access to Covid-19 related medical products. In the preceding plenary debate of 19 May, there was a
lack of
consensus in Parliament over the TRIPS waiver. In the same plenary session, the Parliament also adopted a
resolution on the trade-related impacts and implications of Covid-19 (rapporteur: Kathleen Van Brempt,
S&D, Belgium), urging the Commission to revisit the global framework for IPR, and open a constructive
dialogue on the TRIPS waiver in order to ensure that countries do not face retaliation over Covid-19 related
patent infringements during the pandemic.
The Parliament's resolution (of 20 May 2021) on accelerating progress and tackling inequalities towards
ending AIDS as a public health threat by 2030 also called on the EU to support the TRIPS waiver.
International developments
Third countries’ positions
Several WTO members (Australia, Japan, Norway, Singapore, South Korea, Switzerland and Taiwan) hold
reservations about starting text-based negotiations on a temporary TRIPS waiver. The Ottawa Group
,
including the EU, have put forward a WTO trade and health initiative, which would include trade facilitation
and liberalisation of tariffs for pharmaceutical and medical products. The USA and China have endorsed the
negotiations on the TRIPS waiver on vaccines, but have not mentioned further items of the revised proposal
such as health technologies, therapeutics or diagnostics.
Stakeholder views
The WTO TRIPS Agreement has long been criticised by humanitarian organisations for setting an overly
stringent level of IPR protection in access to medicines, notably in the context of HIV/AIDS and drug-
resistant tuberculosis. Science and research institutes have signed a
statement urging all WTO members to
endorse the TRIPS waiver proposal, including provisions on copyright. Nurses and civil society organisations
including trade unions have urged the 'TRIPS Council' to support the waiver. Proponents have argued the
TRIPS waiver could spur innovation and competition by prompting the sharing of undisclosed information,
while
critics hold that the waiver could disincentivise research and development, and set a precedent that
could in the future deter firms from investing in innovation. The American Chamber of Commerce
(
AmCham) expressed concerns that the waiver could jeopardise vaccine roll-out by diverting raw materials
and disrupting supply chains. The biotechnology industry has questioned the breadth, vagueness and
feasibility of implementing the waiver in national laws across the world.
The revised decision text of the TRIPS waiver proposal and the statement by co-sponsors were presented to the
TRIPS Council on 8-9 June 2021. The EU would need to adopt a common position in the Council of the EU in the
event it decided to support the waiver. In July 2021, WTO members also discussed the alternative EU proposal
for a global trade response for universal vaccination, i.e. limiting export restrictions, support expanding vaccine
production and facilitating the use of existing licensing flexibilities in the TRIPS agreement. The positions remain
divergent and the TRIPS waiver will likely be on the agenda of the next WTO Ministerial Conference in late 2021.
